Floating in Our Food: Food Contact Materials
Introduction
Plastic is everywhere these days — it is an unavoidable fact of life. You put your leftovers in Tupperware, you use a plastic ladle to serve your soup, and you even use plastic cups for your hot coffee. You then clean these out using plastic scrub brushes and other such utensils. We often think about what goes into our food, but how often do we consider what our food goes into?
This is the start of a multi-part series where I will take a look at the research that is currently being conducted around plastic food contact materials (FCMs from here on out). It will be divided into multiple entries. The first of which is this one which will take a look at research into specific types of FCMs and the results they found. The next entry will take a look at the underlying chemistry of plastic FCMs and what happens to them when heat is applied, among other such actions.
What Are Food Contact Materials?
FCMs are essentially what it says on the tin: materials that purposefully contact your food. They can be storage containers, linings, spoons, anything of that sort. The EU defines them as such [1]:
Food contact materials are either intended to be brought into contact with food, are already in contact with food, or can reasonably be brought into contact with food or transfer their constituents to the food under normal or foreseeable use. This includes direct or indirect contact. Examples include:
- containers for transporting food
- machinery to process food
- packaging materials
- kitchenware and tableware
Based on this definition we can safely assume that any item in your kitchen that is even remotely used for preparing or storing food is an FCM, or contains FCMs (I would argue the metal grates in your toaster are the FCM and not the toaster itself, for example).
There are three specific FCMs we will look at in this article. In the next article we will be taking a look at proposed underlying mechanisms for what we observe. These three FCMs are:
- Plastic liners in aluminium cans
- Plastic beverage cups
- Kitchen utensils, in particular ladles and such
What Do You Mean The Liner In My Can?
Aluminium cans contain a plastic-y liner on the inside of them [2]. These can liners may also contain additives such as filaments or pigments, but as the study I am quoting from pointed out these are not a migratory issue as they seem to stay put — the plastic liner itself remains a concern (It should be noted this test was performed at high temperatures.):
Via AF4/MALLS measurements the release of small oligomeric components from internal coating formulations was detected. However, the particle- and element-specific detection system demonstrated the none-migration of nanomaterials (fillers or pigments) from all test samples.
Okay, so what does this mean?
This means that compounds that could have reasonably been made out of smaller repeating units, e.g. possibly a polymer, are found to have migrated into the food simulants the study used in the cans. Any additives added to the liner of the can that were being targeted for study in migration did not manage to move into the food simulant. It should be noted that the food simulant used in this study was an ethanol solution (50% v/v). This is not outside the realm of possibility for real canned food items you could buy at a store (likely a liquor store, but still).
These liners are applied in a plethora of ways. Since we have moved away from Bisphenol A (BPA) the are now more commonly lacquers of some kind of polyester, although it does appear some companies advertise their sprays as BPA Non-Intentionaly Added (BPA-NI)
Plastic Cups?

This cup is polystyrene, but it is here to illustrate a plastic cup. “As seen on American shows like Friends” — https://www.cater4you.co.uk/acatalog/red-plastic-party-cup.html
Studies have shown that heat application to the cups (well above room temperature) have led to contaminant migration into the liquid being held in the cup. This study also used 50% v/v ethanol for its food simulant.
This study also looked at the length of time the preheating was applied and what impact that had on the chemical migrants. From the article:
The observed changes in migration behavior seem to be correlated with the chemical structure of the additives. While an increase of the migration rate with prolongation of the duration of the pre-heating was observed for the higher molecular weight and bulkier additives (FCM №500 and 808), a decrease was observed for the lower molecular weight and linear structure ones (glycerols).
The implication here is that the longer you preheat the cups, the greater the number of larger molecules present migrate into the food simulant. The opposite seems true for the smaller molecules with those being less prevalent the longer you heat the cup. Depending on the molecule you did not have to heat the cup that long:
Two main clusters are clearly observed in the PCA score plot (Fig. 8 — a), distinguishing the non-pre-heated PP cups (blue dashed circled data) and the pre-heated cups (red dashed circle data). Even a 10 min heat-treatment at 40 °C was sufficient to place all substances in a different cluster than the non-pre-heated cups results.
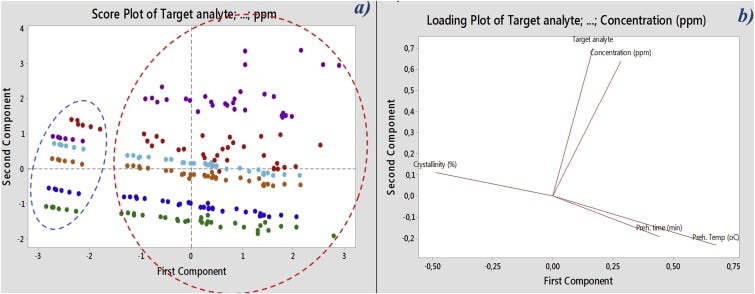
Fig. 8 from the referenced quote above: https://www.sciencedirect.com/science/article/pii/S2214289418303296?via%3Dihub#fig0040
What About Kitchen Utensils?
Yes, even here. Though it should be stated that this depends on the type of plastic that makes up your utensils. This article looked at two specific polymers - PA6 and PA66:
Synthesis of PA6 and PA66: https://journals.plos.org/plosone/article/figure?id=10.1371/journal.pone.0159547.g001
This study used three different food simulants: 20% ethanol, olive oil, and water. Again, the ethanol-plastic interaction is found:
The migration tests suggested that these monomers and oligomers readily migrated into alcoholic beverages possibly due to their features of easy swelling, but hardly migrated into fatty foods possibly due to their low solubility and swelling in the oil. Furthermore, the migrant substances were primarily cyclic monomers and low molecular weight cyclic oligomers. In addition, the PA66-based materials might be easily swelled and degraded, potentially leading to a large amount of PA66 cyclic monomer and oligomers migrating on contact with alcohols at high temperature.
This study performed different tests at different temperature based on the use of the item. Ladles were done near 95–100C, which is when one uses a ladle. As for other items like sesame grinders and cake servers, they tested at 60C — which is clearly above the operating temperature one would expect for a sesame grinder, but a cake server might have a hot cake placed on it.
So, Heat?
Well, it seems that heat certainly does have an impact. How though? Evidence seems to point to the impact of heat on the crystallinity of the polymer, or in the case of ethanol its ability to cause “swelling”. In the next article I plan to explore this on a molecular level.
Bibliography
[1] https://ec.europa.eu/food/safety/chemical_safety/food_contact_materials_en
[2] https://doi.org/10.1016/j.fpsl.2018.03.004